Modifications
- Thread starter Slartibartfast
- Start date
Well, it isn't easy.How do you attach the boat to your bike Slarti ?
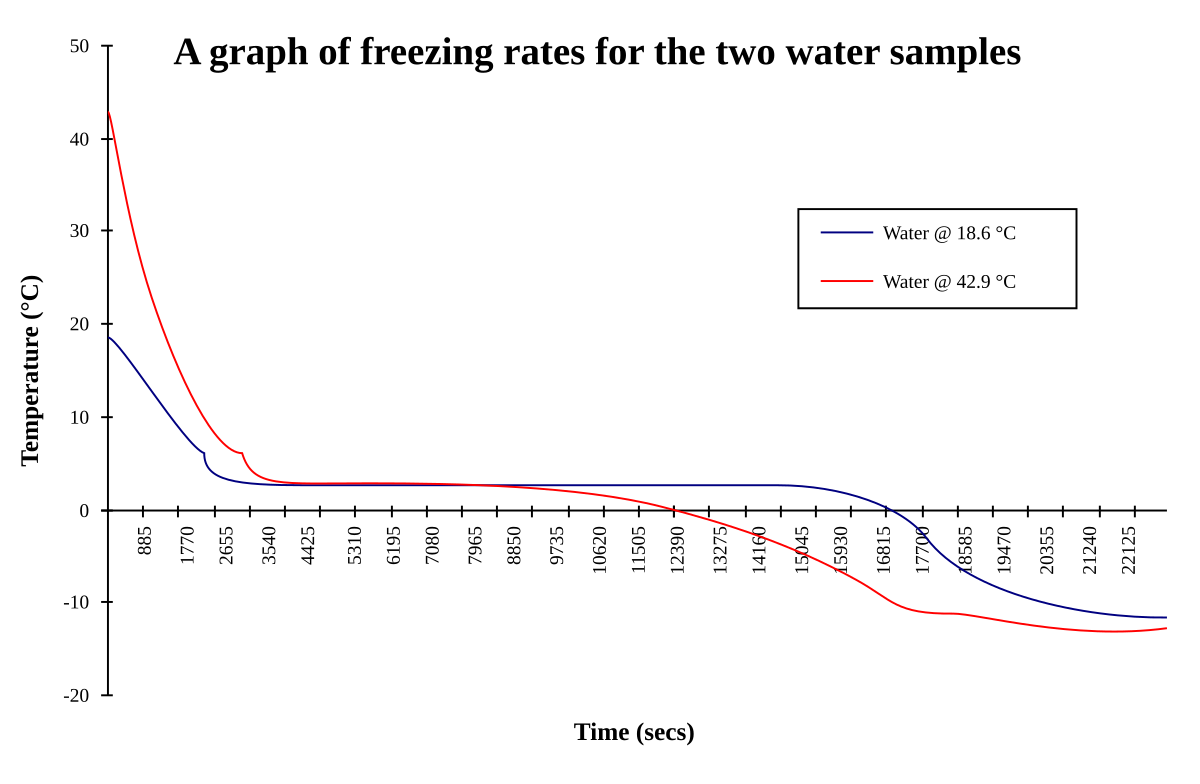
Do you mean a pint of water boiling or a vessel of 1 pint capacity with boiling water in it?Alright....here's one, what freezes quickest a pint of boiling water or a pint of cold water.
No Googling now.
What do you mean by boiling? Do you mean bubbles starting to appear or completely gasified?
D
Deleted member 25121
Guest
Depends on the density of the liquidUK pint = 1lb of liquid.
Trying weighing on your kitchen scales.
Well lets just say a pint of water that's just reached boiling point but no heat is being applied but is still very hot v a pint of cold water straight from your tap.Do you mean a pint of water boiling or a vessel of 1 pint capacity with boiling water in it?
What do you mean by boiling? Do you mean bubbles starting to appear or completely gasified?
At what pressure is it boiling - atmospheric (sea level), vacuum or what?Well lets just say a pint of water that's just reached boiling point but no heat is being applied but is still very hot v a pint of cold water straight from your tap.
You don't get pure water out of your tap. Are we assuming that both samples are pure water?What are you using to freeze the samples. Are you talking about dropping both samples into identical freezers at -32C and seeing which is the first to become solid.
Last edited:
This water freezing thing was something I read years ago, it didn't mention any variables just two masses of water one cold and one very hot, which would freeze first.
I suppose you could say two empty containers, fill one with water from a tap the other water from a kettle that's just come to the boil. Subject them to a below zero temperature at atmospheric pressure...which solidifies fastest, the hot container or the cold ? I could also add 'and why ?'
I suppose you could say two empty containers, fill one with water from a tap the other water from a kettle that's just come to the boil. Subject them to a below zero temperature at atmospheric pressure...which solidifies fastest, the hot container or the cold ? I could also add 'and why ?'
Like everything, external conditions affect how things behave. You also have to be careful about I itial conditions, like what constitutes a pint of boiling water. Say you took two 1 pint beakers of water and boiled one of them. When it's boiling, it'll have a lot less water in it because some will be displaced by the bubbles and the rest will expand to a lower density and some will overflow the rim.
If I can remember my physics lessons correctly in your instance vfr the density wouldn't change because the water was boiling. It's like hard water v soft, hard is very slightly heavier but the density is the same hard or soft.
Back to the which freezes first though, boiling or cold. I suppose most people would say the cold water because it's already part way there, others might say the boiling because it can't be that easy.
The answer is boiling water freezes faster than cold, the reason being some mass would be lost through steam vapour so there's less to freeze.
So now how come a glass of cloudy hard water is no denser than a glass of perfectly clear soft water ?
Back to the which freezes first though, boiling or cold. I suppose most people would say the cold water because it's already part way there, others might say the boiling because it can't be that easy.
The answer is boiling water freezes faster than cold, the reason being some mass would be lost through steam vapour so there's less to freeze.
So now how come a glass of cloudy hard water is no denser than a glass of perfectly clear soft water ?
D
Deleted member 25121
Guest
You're right but there's a lot more to it than that, it's one of those phenomena that are contrary to initial instincts. Here's a good explanation:If I can remember my physics lessons correctly in your instance vfr the density wouldn't change because the water was boiling. It's like hard water v soft, hard is very slightly heavier but the density is the same hard or soft.
Back to the which freezes first though, boiling or cold. I suppose most people would say the cold water because it's already part way there, others might say the boiling because it can't be that easy.
The answer is boiling water freezes faster than cold, the reason being some mass would be lost through steam vapour so there's less to freeze.
So now how come a glass of cloudy hard water is no denser than a glass of perfectly clear soft water ?
Mpemba effect - Wikipedia
 en.wikipedia.org
en.wikipedia.org
Fascinating read that, thanks for posting. I've always seen it as hot water freezing quicker because of evaporation but as you rightly there's a lot more to it than that. Interestingly even now no one really has a definitive answer.
Finally.....when I was a kid many moons ago I use to help out delivering milk for a local dairy, 12 years old and working hard, no EU rules in those days.
I can still remember sitting in an old milk delivery lorry waiting for the dairyman to emerge from a club having dropped off some milk and downed a few free pints while he was at it (sign of the times).
It was raining at the time and I observed rainwater running up, yes up telephone wires not down as you would expect. There's a reason for this phenomena but I can't recall offhand what it is.
I'll search later unless some one beats me to it.
Finally.....when I was a kid many moons ago I use to help out delivering milk for a local dairy, 12 years old and working hard, no EU rules in those days.
I can still remember sitting in an old milk delivery lorry waiting for the dairyman to emerge from a club having dropped off some milk and downed a few free pints while he was at it (sign of the times).
It was raining at the time and I observed rainwater running up, yes up telephone wires not down as you would expect. There's a reason for this phenomena but I can't recall offhand what it is.
I'll search later unless some one beats me to it.
I hate to be the bearer of bad news but I had one of these which I could hear working as the bike thief rode off after cutting a cable lock. Presumably he gave it a good kicking when he got out of sight. Which is what I would have given him if I had been able to run faster. Perhaps I should lay of the cakes and biscuits as suggested elsewhere.Funny you should mention this, been looking at getting one for while but just never got around to it. All sorted now, just ordered it thanks! At £14 if it turns out to be rubbish it's not the end of the world!
I don't expect a lot for £14, my bike is never far from me and I can run so it may just help!I hate to be the bearer of bad news but I had one of these which I could hear working as the bike thief rode off after cutting a cable lock. Presumably he gave it a good kicking when he got out of sight. Which is what I would have given him if I had been able to run faster. Perhaps I should lay of the cakes and biscuits as suggested elsewhere.
That's not right. It takes time for the evaporation to bring the temperature down to the same as the cold water. After that, they're equal. The amount of mass lost by the steam would be insignificant. Try it. Put a mug of boiling water on your kitchen scales and see the difference in weight from initial to cold. You won't even see a gram of difference unless you put a fan on it. The only way the hot water can beat the cold water is by getting better heat transfer. The only way that could happen, assuming all conditions are equal would be if a convection current started, like you have in the oceans. That's feasible as long as you have the right shaped vessel, otherwise the cold will always beat the hot.If I can remember my physics lessons correctly in your instance vfr the density wouldn't change because the water was boiling. It's like hard water v soft, hard is very slightly heavier but the density is the same hard or soft.
Back to the which freezes first though, boiling or cold. I suppose most people would say the cold water because it's already part way there, others might say the boiling because it can't be that easy.
The answer is boiling water freezes faster than cold, the reason being some mass would be lost through steam vapour so there's less to freeze.
So now how come a glass of cloudy hard water is no denser than a glass of perfectly clear soft water ?
D
Deleted member 25121
Guest
That's not what the experiments show, this is the best explanation I could find:That's not right. It takes time for the evaporation to bring the temperature down to the same as the cold water. After that, they're equal. The amount of mass lost by the steam would be insignificant. Try it. Put a mug of boiling water on your kitchen scales and see the difference in weight from initial to cold. You won't even see a gram of difference unless you put a fan on it. The only way the hot water can beat the cold water is by getting better heat transfer. The only way that could happen, assuming all conditions are equal would be if a convection current started, like you have in the oceans. That's feasible as long as you have the right shaped vessel, otherwise the cold will always beat the hot.

Mpemba effect - Wikipedia
 en.wikipedia.org
en.wikipedia.org
Did you read this bit?That's not what the experiments show, this is the best explanation I could find:

Mpemba effect - Wikipedia
en.wikipedia.org
"There exists a set of initial parameters, and a pair of temperatures, such that given two bodies of water identical in these parameters, and differing only in initial uniform temperatures, the hot one will freeze sooner "
There exists a set of parameters where it can happen doesn't mean that it will happen when you try it. Basically, there is one set of parameters where it can happen and thousands of sets where it won't.
Later in that article:
"In 2016, Burridge and Linden defined the criterion as the time to reach 0 °C (32 °F), carried out experiments and reviewed published work to date. They noted that the large difference originally claimed had not been replicated, and that studies showing a small effect could be influenced by variations in the positioning of thermometers. They say, "We conclude, somewhat sadly, that there is no evidence to support meaningful observations of the Mpemba effect".
water freezes around seed crystals - like Champagne bubbles."There exists a set of initial parameters, and a pair of temperatures, such that given two bodies of water identical in these parameters, and differing only in initial uniform temperatures, the hot one will freeze sooner "
If you have very clean water, with little dissolved salts (eg carbonates and oxides of magnesium, calcium, iron etc), then at below a certain concentration, the movement of those seeds become much more important, causing the previously hot water container (in which the seeds move faster and are better dispersed) to freeze at normal temperature (which is zero Celsius at 1 atm) while the colder water will go supercooled without freezing.
BTW, you cannot derive 'free energy' from this.
Last edited:
Can you get free water by planting the seeds? I'll look out for them at the garden centre next time I go there for my virgin boy eggs.water freezes around seed crystals - like Champagne bubbles.
If you have very clean water, with little dissolved salts (eg carbonates and oxides of magnesium, calcium, iron etc), then at below a certain concentration, the movement of those seeds become much more important, causing the previously hot water container (in which the seeds move faster and are better dispersed) to freeze at normal temperature (which is zero Celsius at 1 atm) while the colder water will go supercooled without freezing.
BTW, you cannot derive 'free energy' from this.
D
Deleted member 25121
Guest
Yes, it's rather badly worded isn't it. The effect has been widely observed since the days Aristotle, so there are clearly many sets of initial parameters where the effect is observed, many "thousands" even.Did you read this bit?
"There exists a set of initial parameters, and a pair of temperatures, such that given two bodies of water identical in these parameters, and differing only in initial uniform temperatures, the hot one will freeze sooner "
There exists a set of parameters where it can happen doesn't mean that it will happen when you try it. Basically, there is one set of parameters where it can happen and thousands of sets where it won't.
So their results invalidate the research of many others eh? That's not quite how scientific method works, try Googling "Mpemba effect" there are lots of write-ups about it.Later in that article:
"In 2016, Burridge and Linden defined the criterion as the time to reach 0 °C (32 °F), carried out experiments and reviewed published work to date. They noted that the large difference originally claimed had not been replicated, and that studies showing a small effect could be influenced by variations in the positioning of thermometers. They say, "We conclude, somewhat sadly, that there is no evidence to support meaningful observations of the Mpemba effect".
the idea of using seeds is not far fetched.Can you get free water by planting the seeds?
The Star Wars 'vaporator' may be one of those water collector working on this sort of idea.
Air containing a little humidity is cooled, releasing water. You need seeds, around them the water droplets form.
Related Articles
-
 MTF Enterprises announces acquisition of EMU Electric Bikes
MTF Enterprises announces acquisition of EMU Electric Bikes- Started by: Pedelecs
-
 Wisper 806T folding bike wins Which? ‘Best Buy’
Wisper 806T folding bike wins Which? ‘Best Buy’- Started by: Pedelecs
-
 Sustrans calls for protected cycle lanes
Sustrans calls for protected cycle lanes- Started by: Pedelecs
-
 Amazon launch their first UK e-cargo micromobility hub
Amazon launch their first UK e-cargo micromobility hub- Started by: Pedelecs



